
Product safety is one of the driving factors in government oversight, as applied to the consumer. Whether it is concerned with medications, food, transportation, or housing, product safety drives the development of regulations for manufacturing and production across industries. As a result, the consumer is better assured of their purchase or medical treatment, feeling more secure that something terrible isn’t going to happen, at least due to product quality. That assurance is printed on the packages as nutritional labeling, assays for medications, or other means. The intent is to trust the information provided so that when you reach for a pain reliever that lists the strength of the medicine, you can feel confident that it is correct.
What is that confidence based on? It isn’t trusting producers will “do the right thing”; it’s because someone is methodically monitoring the producers’ activities to ensure they are “doing the right thing” and being held accountable. This article will look at the guidelines that apply to laboratory inspections, the common problems that arise (where information exists), and the role that laboratory informatics can play in making compliance easier.
The basis for laboratory audits and inspections
Within laboratories, the point of guidelines is to ensure the validity of laboratory results, i.e. that the accuracy and integrity of the data and information provided are high. One thing you will notice as we list and review the various guidelines is that they are often drawn from documents applied to manufacturing/production facilities. For example:
- Regulations, like 21 CFR Part 11, created by entities such as the Food and Drug Administration (FDA);
- Standards, like the ISO 9000 family, created by groups such as the International Organization for Standardization (ISO), and,
- Guidance, on topics like Good Automated Manufacturing Practice (GAMP), created by entities such as the International Society for Pharmaceutical Engineering (ISPE).
The concern here is for products that reach the consumer rather than those currently in the research and development (R&D) phase of their operations, although those should apply the guidelines to their work. One exception is the FDA, which conducts lab inspections for labs involved with clinical trials, or studies in organisms when the results of that work will be used in FDA approvals for medical products as they move through the clinical trials/approval process.
Other regulations, standards, and guidelines help ensure the integrity of lab results when analytical data and information is made available to the public, for example, via water quality testing. These include:
- Regulations developed by the Environmental Protection Agency (EPA);
- The Centers for Medicare & Medicaid Services (CMS) “Standards and Certification: Laboratory Requirements” (42 CFR 493) developed by Centers for Medicare & Medicaid Services (CMS) for Clinical Laboratory Improvement Amendments (CLIA) Laboratories https://www.cdc.gov/clia/index.html
- Standards like ISO/IEC 17025 developed by the ISO; and
- Guidelines and accreditation requirements developed by the American Association for Laboratory Accreditation (A2LA).
Beyond this, the Occupational Safety and Health Administration (OSHA) has standards to protect laboratory workers.
Note: The guidelines we are considering here are for overall lab operations and differ from specific test guidelines/methods produced by the ASTM[1], United States Pharmacopeia (USP)[2], and other standards/regulatory bodies.
When we consider lab inspections, several questions need to be answered: What laboratory activities and functions are being evaluated? What are the guidelines that are being used? What types of problems are being uncovered? We’ll address those questions now.
What laboratory activities and functions are being evaluated?
As noted earlier, the purpose of the inspection process is to ensure the results of laboratory work are on a solid footing and to uncover areas where things need to be improved to ensure the results are reliable. That is the basis for including a requirement for a validation process to produce documented proof that something works as intended and is needed. The areas that need to be addressed fall into the following categories, as shown in Figures 1 and 2.
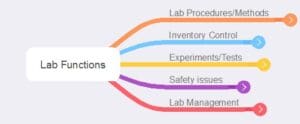
Figure 1 – Summary of basic categories of lab functions
Figure 2 – Fully expanded lab function diagram. The diagram will be difficult to read, so the individual sections are split out in Figures 3-7.
The point of including this diagram is to show at least some of the interconnections between functions. A complete set of interconnections would be challenging to view, but most should be easily understood.
The list of functions is not intended to be a detailed depiction of the subject but one common to many industries. Some industries may expand or edit points, for example, organism handling.
One thing to note: these are not depictions of informatics systems but of lab functions devoid of software considerations. A given software application may cover multiple areas totally or partially. It is up to you to decide how to partition functions and deal with overlapping service offerings. Some might be specific to one lab, and others, such as inventory control, address the needs of several labs.

Figure 3 – Laboratory procedures management
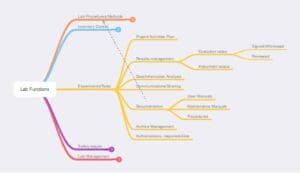
Figure 4 – Experiments and testing. These are descriptions of activities without considering whether they occur in research or service labs. In an implementation devoid of software, it doesn’t matter, but a distinction would be created if the lab used an electronic lab notebook (ELN) or laboratory information management system (LIMS).
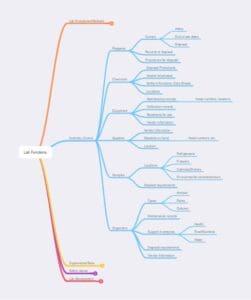
Figure 5 – Inventory control, as noted earlier, may be within one lab or span the needs of a multi-lab facility. Some functions, such as sample locations, could be duplicated in an ELN or LIMS
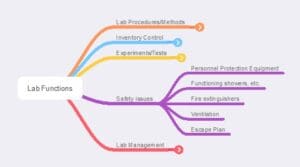
Figure 6 – Laboratory safety functions. Lab personnel should be aware of what these items are, how they are to be used, and how to test if they are functional (this may be a facilities management responsibility)
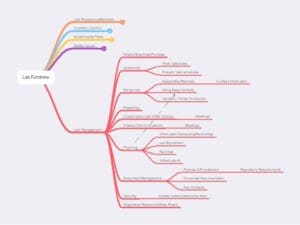
Figure 7 – Lab Management functions
What are the guidelines that are being used?
The inspection guidelines will vary by industry. For example, pharmaceutical and biotech industries are thoroughly covered by the FDA, an organization that has the authority to bring research and production operations to a halt if serious issues are encountered. Stopping the production of a pharmaceutical plant is a significant occurrence. Once the problem has been corrected, the plant may require cleanup to bring it back to normal operating conditions. Other industries, such as independent testing labs in the environmental sector, may be affected by regulations, standards, and guidelines from the EPA and ISO, with inspections performed by the A2LA. While listing organizations and their guidelines, a given industry or lab may be affected by requirements from multiple groups. The policies cover the same materials for many issues, but you should be aware that unique needs can exist. The following table will show the responsible agency and reference documents for guidelines. Note that some documents focus on the audit/inspection process rather than the requirements of the full guideline.
| Agency | Reference Documents |
| FDA | The first document points to the guidelines, and the next two are current topics of interest. The last bullet is the original regulation published in the Code of Federal Regulations.
· Compliance Policy Guide: https://www.fda.gov/regulatory-information/search-fda- guidance-documents/guidance-industry-q7a-good-manufacturing-practice-guidance- active-pharmaceutical-ingredients · Readiness: https://www.intouch-quality.com/blog/fda-inspection-readiness-what-to- expect-and-how-to-prepare · Data Integrity: https://www.rdworldonline.com/data-integrity-compliance-with-gmp-and- fda-requirements/ · 21 CFR Part 11: |
| EPA | Good Laboratory Practices Standards Compliance Monitoring Program https://www.epa.gov/compliance/good-laboratory-practices-standards-compliance-monitoring- program
On April 8, 1995, the EPA issued a letter[3] “Guidelines for Study Rejection Based on GLP Considerations” that emphasizes the importance of certain aspect of the GLP guidelines. |
| ISO- 9000 | · ISO 9001 Audit Checklist for Laboratories: https://advisera.com/9001academy/blog/2018/09/04/iso-9001-audit-checklist-for- laboratory/
· Checklist of Mandatory Documentation Required by ISO 9001-2015: https://advisera.com/9001academy/knowledgebase/list-of-mandatory-documents- required-by-iso-90012015/ |
| ISO- 17025 | · LIMS guide for ISO/IEC 17025 Laboratories: https://www.limsforum.com/ebook/lims- selection-guide-for-iso-iec-17025-laboratories/
· ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories https://www.iso.org/files/live/sites/isoorg/files/store/en/PUB100424.pdf Note: this is a very broad overview, however the “For more Information” section is useful · ISO/IEC 17025 on Wikipedia: https://en.wikipedia.org/wiki/ISO/IEC_17025 |
| A2LA | The A2LA and accreditation: https://a2la.org/accreditation/
Since this group covers several industries, the recommendation is to visit their site for items that pertain to you and your lab. For example their work includes, but is not limited to, the following programs: |
| · ISO 15189 and CLIA for clinical testing laboratories
· ISO/IEC 17020 for inspection bodies · ISO/IEC 17043 for proficiency testing providers · ISO/IEC 17065 for product certification bodies · ISO 17034 for reference material producers · ISO 20387 for biobanking facilities · ISO/IEC 17025 for testing and calibration Laboratories
In addition, the following article should prove useful for understanding the role of A2LA: https://www.labmanager.com/ask-the-expert/laboratory-accreditation-101-23168 |
|
| OSHA | · OSHA – Laboratories – Enforcement:
https://www.osha.gov/laboratories/enforcement · About OSHA’s Laboratory Standard: https://www.vumc.org/safety/osha/lab-standard |
| CLIA | · CLIA: https://www.cdc.gov/clia/index.html |
| ISPE | The ISPE provide several standards concerning Good Automated Manufacturing Practices (GAMP) that address laboratory operations: https://ispe.org/initiatives/regulatory/what-gamp Within their range of activities, the following publications will assist in managing compliance and preparing for inspections and audits:
· GAMP Good Practice Guide: A Risk-Based Approach to Compliant GxP Computerized Systems · GAMP Good Practice Guide: Calibration Management · GAMP Good Practice Guide: Electronic Data Archiving · GAMP Good Practice Guide: Global Information Systems Control and Compliance · GAMP Good Practice Guide: IT Infrastructure Control and Compliance · GAMP Good Practice Guide: Testing of GxP Systems · GAMP Good Practice Guide: Validation of Laboratory Computerized Systems · GAMP Good Practice Guide: Validation of Process Control Systems |
What types of problems are being uncovered?
The analysis of problems uncovered during inspections comes largely from industries covered by the FDA and its guidelines in Form 483 letters[4] sent to organizations. These can cover all aspects of a company’s operations, though as noted earlier, enforcement is usually confined to production operations and potential products being considered for clinical trials. A recent (2022) American Pharmaceutical Review article[5] asks: “Why are the top 10 FDA Form 483 issues mostly the same for the past twenty-three years? What is/are the cause(s)?” Among the problems this article uncovered for lab operations are:
- Testing: “Testing and release of products for distribution do not include appropriate laboratory determination of satisfactory conformance to the (final specifications) (identity and strength of each active ingredient) prior to release”;
- General requirements: No details provided;
- Stability: “Stability program – there is no written testing program designed to assess the stability characteristics of drug products.”
- System controls: No details provided;
- Method validation: Found lacking;
- Sample management: More controlled;
- [Materials/testing] out of specification (OOS) / out of trend (OOT).
Beyond that, the article notes that many of these failures are the result of the inadequate application of technologies. The author notes “many small firms are still paper-based and do not have the necessary money and technology to analyze process and analytical data in such a way that allows for proactive responses to trends.”
A second article entitled “Data Integrity: 2020 FDA Data Integrity Observations in Review”[6] discussed failures of data integrity, an important topic over the last few years. Among the concerns it covers are:
- Deletion or manipulation of data,
- Aborted sample analysis without justification,
- Invalidated OOS results without justification,
- Destruction or loss of data,
- Failure to document work contemporaneously, and
- Uncontrolled
While the points above we specifically addressed within the pharma/biotech industries, there is no reason to assume that they are limited to those fields. The same problems can likely be found in any industry. Analysis of Form 483s is often done because of the importance of the information they contain, and their availability through the Freedom of Information Act with some redactions.
Can laboratory informatics help address these issues?
Within the realm of guidelines covered in this piece, the dominant activity within the lab is testing and activities that support service laboratory operations. The service lab is common in most industries. The details of sample types and testing will vary, but the operational behavior will be the same and will work like this:
- Samples are submitted for testing. In many labs, these are done on paper forms listing sample type, testing to be done, whom to bill, and a description of the sample and any special concerns or issues. In labs with a laboratory information management system (LIMS), this can be done online by lab personnel or the sample submitter.
- The work is logged in, and rush samples are brought to management’s
- Analysts generate worklists and perform the analysis required, and results are recorded in the LIMS or in lab notebooks.
- The work is reviewed and approved for release and, in paper systems, recorded on the submission forms.
- Reports are sent to whoever submitted the work, either electronically or via the method the submitter requested.
LIMS are designed to carry out this work and have facilities to solve many of the issues noted as failures in the previous section. For example, if you look at the elements discussed under the topic of data integrity, the top four bullets shouldn’t occur. LIMS do not let you delete data although data can be edited. If it is edited, a reason must be given for the change, who made the change must be documented, and when the new data is entered it shouldn’t overwrite the old data but be maintained as part of an audit trail. If questions arise about changes to data, the history is preserved within the LIMS. Many of these problems can still occur with spreadsheet implementations of sample tracking, but a LIMS installation will avoid any of these issues.
LIMS often have built-in document management facilities, so documentation loss is prevented, and the material is easily searched. Documentation of tests and exception reporting is also a feature of LIMS. A specific program to support stability testing, one of the failure points noted, can be incorporated as part of the software.
The introduction of laboratory informatics can streamline lab operations by providing a single point of access to look up documentation, procedures, schedules, test requirement, test results, trends in data, flagged out-of-spec samples, and so on. It can end the paper chase in lab operations.
In closing…
Laboratory inspections, planned and surprised, are an ongoing fact of life. The issue is not to prepare for them but to always be prepared for them. A well-designed LIMS, like LabLynx ELab LIMS, can give you control over lab operations, documentation, testing, test results, equipment calibration schedules, and more.




