
It’s Thursday morning, and the director of R&D has a rush set of samples that need to be analyzed now (it’s always a rush). Do you have the materials on hand to do the work? Who’s your go-to supplier for fast delivery if something is in short supply? Is the equipment that will be used in good working condition and recently calibrated?
Did you buy something only to discover you already had two of them?
Did you know you had a reagent but needed to know where it was?
Did you find a stock of reagents in cabinets well past their use-by date? How do you dispose of them?
Have materials gone missing or been used up without anyone knowing it?
Inventory management is one of the routine aspects of lab work. Still, the level of interest in it will rise considerably if any of the questions above, or others, don’t have a ready answer. Inventory management is a significant factor in ensuring that lab work is done with as little chaos or disruption to the normal workflow as possible. This article will look at some characteristics of an inventory management system as a starting point for implementing one or using it to improve lab operations. We’ll examine what we have to manage, how security fits in, what implementation considerations exist, and what inventory management requirements should be considered.
Benefits of an inventory management system
Improving productivity and workflow
Electronically finding what you need when you need it can save many person-hours, avoiding having lab personnel spend time physically searching for materials. (It’s even worse when you can’t locate those materials). Another issue arises when having to delay a procedure because reagents need to be prepared to the correct concentration. What if you find out that the reagent calibration needs to be redone because the current one is outdated?. All of these complications comes down to wasted time or delays in getting work done.
Ideally, you’d like to be able to look at the work planned for the next week, determine the materials required, and then either verify that they are available or order whatever is needed so that work can progress at a smooth pace.
Minimizing stock-level issues
Having too much of an item means resources have been spent when additional quantities of the item were not needed. Not enough of an item means work is delayed unnecessarily. Either of these can give a lab the appearance of needing better management.
Compliance with safety standards (corporate and municipal)
Safety standards can come from a variety of sources: corporate, municipal, Occupational Safety and Health Administration (OSHA)[1], and industry groups. One of the critical components of these standards is having knowledge of what materials are in a facility, where they are, and the safety information about them (e.g., flammability, toxicity). These can include dangerous liquids, solids, and compressed gases in a laboratory setting. For example, in a fire, first responders must know what they are facing, where materials are, and any special considerations in dealing with problems.
Reliability of information
With proper security measures (covered in more detail below), an inventory management system can provide chain-of-custody information about materials and samples, detailing who had access to them and who was using them. Another issue is that of audits of materials, reagents, and samples. Again, demonstrating control over these elements is critical to successfully managing a laboratory and avoiding problems like issuing complaint letters from regulatory agencies.
What materials do we have to manage?
If you start thinking about it, there is a lot of inventory to be tracked and maintained, particularly if you are in a regulated industry and are being held accountable for the lab’s operation by an outside agency (as well as corporate management). Table 1, below, provides examples of materials that need to be managed in the lab and what considerations should be made for those materials.
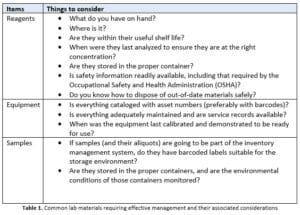
In addition, the lab must keep track of items such as personal protective equipment (PPE) and cleaning supplies.
Facilities considerations
The size of the organization matters. In a facility with multiple laboratories, inventories can be managed differently with considerations for the individual labs and their common needs. Should a central storage area for commonly used reagents and other consumables be implemented? That organization can make a lot of sense from the standpoint of efficient organization and safety. For example, if hazardous materials are being stored, the storage area can be partitioned such that ventilation and fire suppression equipment can be concentrated in those areas. This should streamline the ordering of materials, avoiding having too much infrequently used (and potentially hazardous) material on hand, while also ensuring that the right quantities of needed materials are available.
In addition, some common considerations for equipment such as biobanking freezers and incubators may be made. Note that gathering freezers and incubators into a common area may make it easier to monitor them and provide backup power if needed.
While a central depot for accessing materials would be useful, each laboratory may require its own inventory management system focused on the activities specific to those labs. Testing labs will have their own sets of samples that need to be maintained, as well as prepared reagents used in procedures.
Given the considerations in the previous paragraphs, there may be a need for two inventory management systems: a general facilities-wide system that addresses needs common to all labs and individual lab-specific systems that could be addressed by a laboratory information management system (LIMS) or electronic laboratory notebook (ELN).
Computer-based inventory management systems require the same management concerns as any other system. Protection against power failure (including battery backup and generators) is needed to help ensure the integrity of the application and its data. Periodic system backups are required, as are well-documented and tested recovery procedures. Those using the system(s) need to be educated in their use with operational procedures and who is responsible for the operation and maintenance of the system clearly laid out.
Materials labeling, personnel identification, and security
One problem common to inventory management systems is data entry which occurs when materials are removed, added, or changed locations (note: locations are another data element). The most common method of reducing errors in data entry for inventory management systems is using barcodes and barcode scanners. These can be used singly or in combination. For example, a chemical reagent may have a standard one-dimensional alphanumeric barcode for identification of the material, and a QR-code[2] pointing to a safety information document stored on a server. A barcode on the shelf would be used to note the location.
Barcode materials must be chosen to suit their use. While paper may work for many cases, cold storage locations would require special barcode materials (cryogenic thermal-transfer labels) suited to those environments.
Barcodes and RFID chips can be used in storage areas to track who enters the space. Barcodes require active participation in scanning badges, and RFID chips can be automatically scanned when someone enters or leaves the inventory area. This provides a log of who has had access to materials and what may have been removed, providing a measure of security over samples and materials. Organizations that require more stringent security may use a biometric scanner (e.g., fingerprint) to open and close doors upon entry or exit.
Environmental monitoring
Monitoring the environmental conditions for reagents and samples is a matter that requires some attention. Many samples are sensitive to changes in temperature, humidity, and light. In addition, samples in biobanks are going to be particularly sensitive to those factors as well as power outages. Networked components can monitor these conditions, periodically log the status of sensors, and provide warnings (e.g., email, text, automated calls to your phone) when conditions are outside expected ranges. You can also monitor inventory locations for water leaks, provide alerts, and automatically shut off water supplies to avoid damage. Monitoring with logging is needed to show that nothing happened to adversely affect samples and raise concerns about the validity of the work performed on them.
In closing…
An inventory management system has moved from the “nice to have” to the “required” category of laboratory informatics along with LIMS, ELNs, and instrument data systems. By managing access to reagents, samples, and equipment, they can ensure that those elements of lab operations are readily available and in good working order and that there is good, demonstrable, control over chain-of-custody for critical samples and materials used in lab procedures. Inventory management systems can help ensure that the normal laboratory workflow can proceed without being disrupted by a lack of needed components, helping keep the pace of operations on schedule.
Inventory management system capabilities aren’t limited to dedicated stand-alone software; a LIMS will often have these features built-in. LabLynx ELab LIMS software solution is one example of a product that encompasses both full LIMS functionality as well as inventory management in one package.
References
[1] http://osha.gov [2] QR-code – “QR” stands for quick-response and can contain a complete URL




